
For example the oxidation of carbon to form CO 2 and the reduction of carbon by the addition of hydrogen to yield methane (CH 4 ).These reactions are called Redox reactions (reduction-oxidation) which are chemical reactions in which atoms have their oxidation number changed.
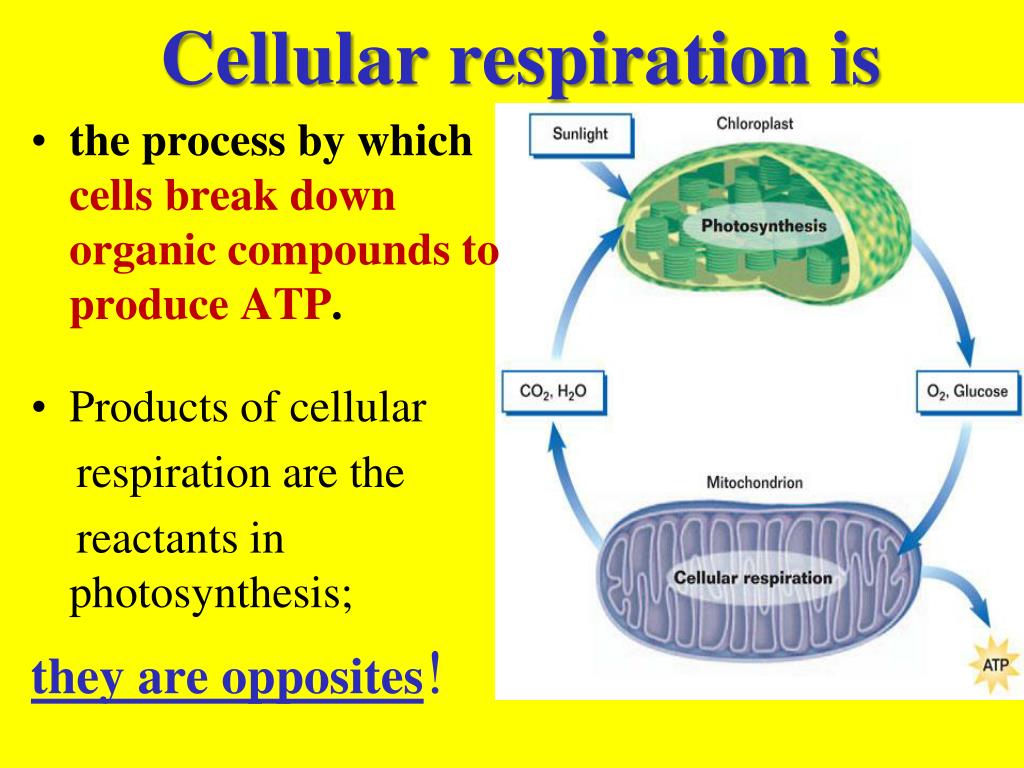
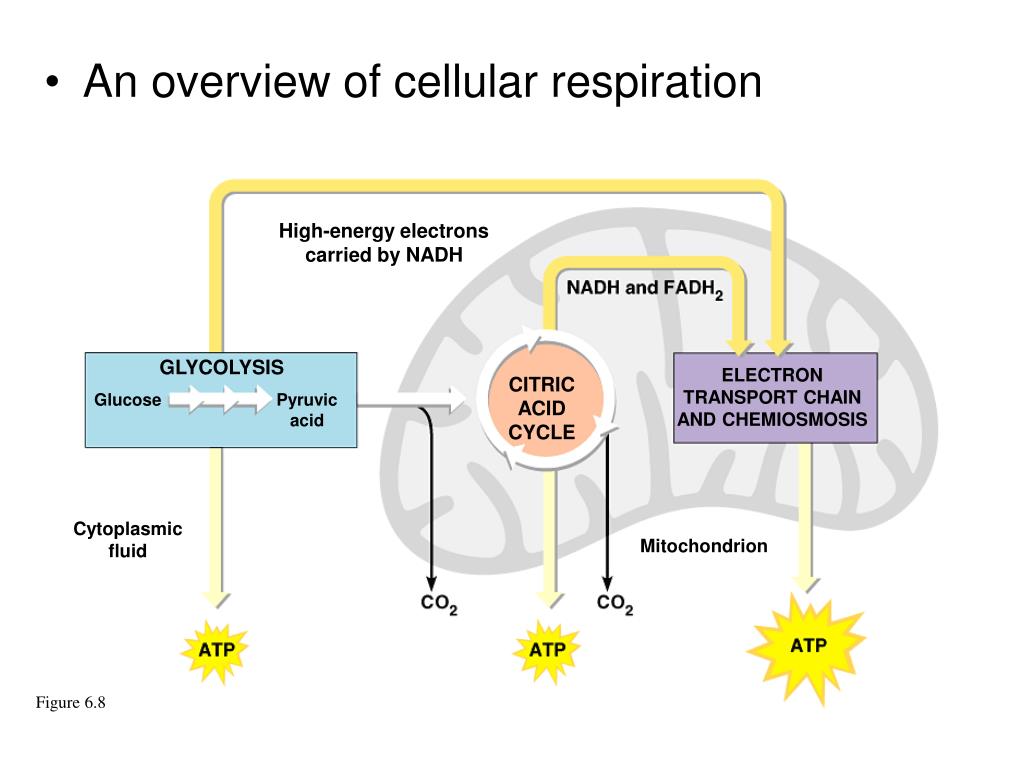
Reduction involves the gain of electrons through the addition of hydrogen or the loss of an oxygen molecule.Oxidation involves the loss of electrons from an element through the gain of oxygen or the loss of hydrogen.Aerobic respiration involves the complete breakdown of organic molecules for a larger yield of ATP (oxygen is required) Anaerobic respiration involves the incomplete breakdown of organic molecules for a small yield of ATP (no oxygen required). The final result is: C 6H 12O 6 (glucose) + 6O 2 → 6H 2O + 6CO 2 + 38ATP molecules.Cell respiration is the controlled release of energy from organic compounds to produce ATP. Without oxygen altogether, electron transport and proton pumping would come to a halt.ĬELLULAR RESPIRATION IS SYNTHESIS OF ATP. Thus, pyruvic acid does not enter the Krebs cycle, “backs up” in the system, and ferments to form lactic acid. When there is inadequate oxygen, such as during anaerobic exercise, electron transport does not keep up with the breakdown of glucose into pyruvic acid. The protons (H +) are picked up by the negatively charged oxygen molecules, two protons per molecule, to yield water, H 20. The protons provide the energy for the formation of 34 ATP molecules. the concentration of H + is much higher in the outer compartment. Hydrogen protons diffuse from the outer compartment back into the inner compartment, as a result of a pH gradient established by the earlier proton pumping action, i.e. STEP 4 : Chemiosmosis (oxidative phosphorylation). The electrons flow by virtue of the presence of oxygen at the end of the transport sequence, wherein each oxygen molecule picks up two electrons and becomes negatively charged (-2). The accumulated electrons move across the cristae of the mitochondria, which are part of the inner compartment (matrix) membrane, creating an electrical current, which pumps the accumulated H + out of the inner compartment into the outer compartment. Pyruvic acid goes through an elaborate oxidative process, in the mitochondria of cells, resulting in many more electrons and protons, two more ATP molecules, and carbon dioxide (also generated during the transition from glycolysis). The bicarbonates are then restored for further buffering of acids. Lactic acid is eventually utilized in the resynthesis of glucose, or it is oxidized into H 2O and CO 2. Lactic acid is buffered (neutralized) by bicarbonates (controlled by the kidneys). Thus, glucose utilization in the absence of oxygen, anaerobic glycolysis, is highly inefficient. Only five percent of the total energy created during cellular respiration is generated at this time, two ATP molecules of a total of 38. Although glycolysis does not require oxygen, when inadequate oxygen is available to the mitochondria, some of the pyruvic acid does not enter the mitochondria, but rather breaks down into lactic acid, a process known as fermentation. In cell cytoplasm, glucose is broken down ( oxidized) into electrons, hydrogen protons (H +), and pyruvic acid, most of which enter the Krebs cycle ( aerobic) in the mitochondria of cells. Glucose (sugar) is broken down ( oxidation) to supply energy for cellular respiration. Cellular respiration is the utilization of oxygen for the synthesis of ATP. Cells create energy by breaking down adenosine triphosphate, ATP molecules.


 0 kommentar(er)
0 kommentar(er)
